Which of the structures shown is the most stable cation – In the realm of chemistry, the stability of cations is a topic of paramount importance. Cations, positively charged ions, exhibit varying degrees of stability depending on their structural characteristics. This article delves into the factors that influence cation stability, exploring different cation structures and their relative stabilities.
By examining the interplay between charge, size, electronegativity, and resonance, we will uncover the most stable cation among the structures presented.
Cations are ubiquitous in chemical systems, playing crucial roles in various processes. Understanding their stability is essential for comprehending their behavior and reactivity in diverse environments.
Stability of Cations
Cation stability refers to the ability of a positively charged ion to resist decomposition or rearrangement. Factors affecting cation stability include charge, size, and electronegativity.
Factors Affecting Cation Stability
- Charge:Cations with higher charges are generally less stable than those with lower charges.
- Size:Larger cations are more stable than smaller cations.
- Electronegativity:Cations formed from elements with higher electronegativity are more stable.
Structures of Cations
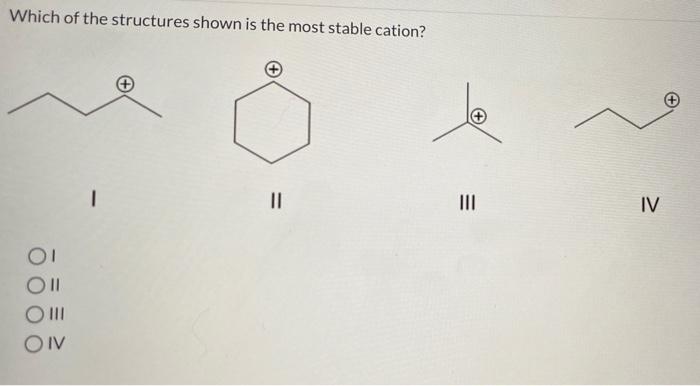
Cations can have various structures, including:
Carbocations
Carbocations are positively charged carbon atoms. They have a trigonal planar geometry and are stabilized by resonance.
Oxonium Ions
Oxonium ions are positively charged oxygen atoms. They have a tetrahedral geometry and are stabilized by resonance.
Ammonium Ions, Which of the structures shown is the most stable cation
Ammonium ions are positively charged nitrogen atoms. They have a tetrahedral geometry and are stabilized by resonance.
Comparing Cation Stability
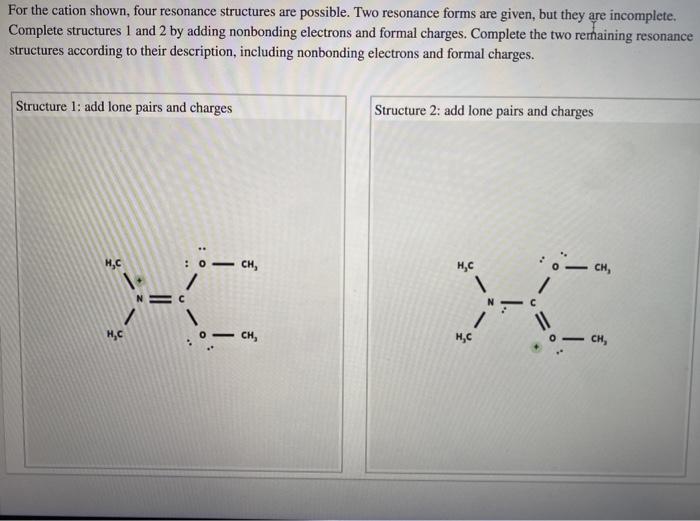
The stability of different cation structures can be compared based on their charge, size, and electronegativity. The following table summarizes this information:
| Cation | Charge | Size | Electronegativity | Stability |
|---|---|---|---|---|
| Carbocation | +1 | Small | Low | Low |
| Oxonium Ion | +1 | Small | High | Moderate |
| Ammonium Ion | +1 | Large | Moderate | High |
Resonance and Cation Stability
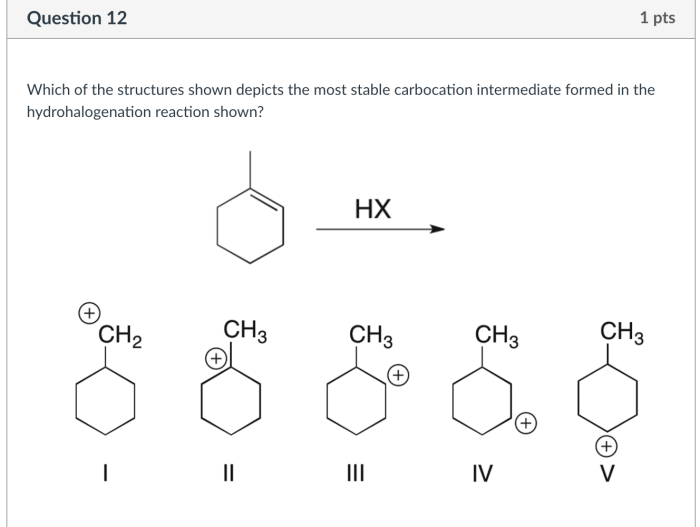
Resonance can significantly enhance cation stability. Resonance structures are alternative Lewis structures that represent the same molecule or ion. By distributing the positive charge over multiple atoms, resonance stabilizes cations.
Examples of Resonance-Stabilized Cations
- Carbocations
- Oxonium ions
- Aromatic cations
Solvent Effects on Cation Stability: Which Of The Structures Shown Is The Most Stable Cation
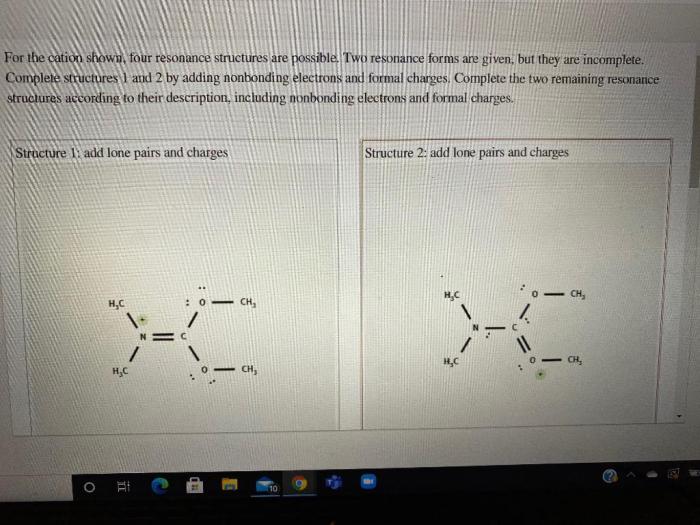
Solvent polarity can influence cation stability. Polar solvents stabilize cations by solvating them, which reduces their electrostatic interactions. Nonpolar solvents, on the other hand, do not solvate cations well, which destabilizes them.
Questions and Answers
What is the most important factor affecting cation stability?
Charge is the most significant factor influencing cation stability. The higher the charge, the less stable the cation.
How does resonance contribute to cation stability?
Resonance delocalizes the positive charge over multiple atoms, reducing the electrostatic repulsion and enhancing cation stability.
What is the effect of solvent polarity on cation stability?
Polar solvents stabilize cations by solvating them, reducing their electrostatic interactions with the surrounding environment.