Chemistry the molecular nature of matter and change 9th edition – Embark on a captivating journey with Chemistry: The Molecular Nature of Matter and Change, 9th Edition, an authoritative guide that unravels the intricate world of chemical principles. Delve into the fundamental concepts that govern matter and energy, explore the organization and properties of elements, and witness the dynamics of chemical reactions.
Prepare to be enthralled as we delve into the fascinating realm of chemistry.
Our exploration continues with a deeper understanding of states of matter, intermolecular forces, and the properties of solutions. Thermodynamics and kinetics take center stage, revealing the laws that govern chemical reactions and their rates. Equilibrium and Le Chatelier’s principle provide insights into the delicate balance of chemical systems, while acids, bases, and salts showcase their ubiquitous presence in our world.
Finally, we venture into the realm of nuclear chemistry, unlocking the secrets of atomic nuclei and their applications.
1. Key Concepts in Chemistry: Chemistry The Molecular Nature Of Matter And Change 9th Edition

Chemistry is the study of matter and its changes. It is a fundamental science that has applications in many fields, such as medicine, engineering, and environmental science. The key concepts in chemistry include the structure of matter, the properties of matter, and the changes that matter can undergo.
Matter and Energy
- Matter is anything that has mass and takes up space.
- Energy is the ability to do work.
- Matter and energy are related by the equation E=mc², where E is energy, m is mass, and c is the speed of light.
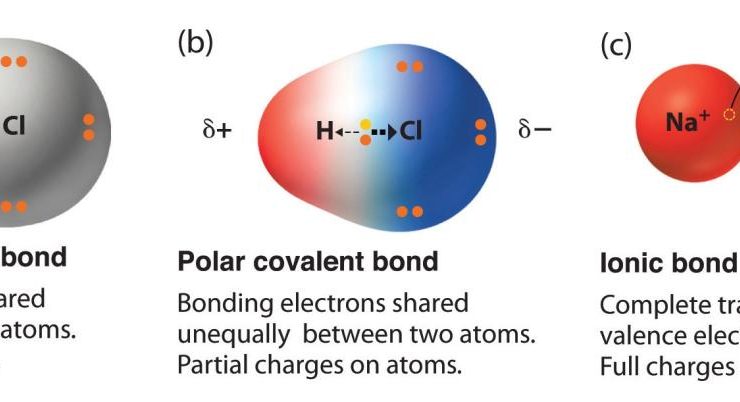
Chemical Bonding
- Chemical bonding is the force that holds atoms together to form molecules.
- There are different types of chemical bonds, including ionic bonds, covalent bonds, and metallic bonds.
- The type of chemical bond that forms depends on the properties of the atoms involved.
Molecular Structure
- Molecular structure is the arrangement of atoms in a molecule.
- The molecular structure of a molecule determines its properties.
- For example, the shape of a molecule affects its polarity, which in turn affects its solubility.
2. The Periodic Table and Chemical Properties

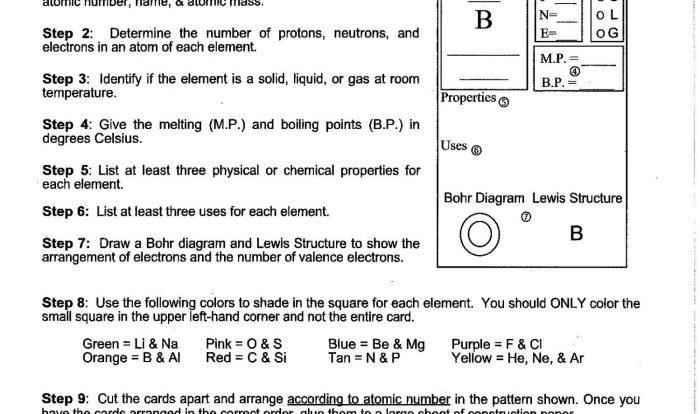
The periodic table is a tabular arrangement of the chemical elements. It is organized by atomic number, which is the number of protons in the nucleus of an atom. The periodic table can be used to predict the properties of elements and to understand the relationships between them.
Organization of the Periodic Table
- The periodic table is organized into 18 vertical columns, called groups, and 7 horizontal rows, called periods.
- Elements in the same group have similar chemical properties.
- Elements in the same period have the same number of electron shells.
Properties of Elements, Chemistry the molecular nature of matter and change 9th edition
- The properties of elements are determined by their atomic structure.
- The atomic structure of an element includes the number of protons, neutrons, and electrons in the atom.
- The number of protons in the nucleus determines the element’s atomic number.
Periodic Trends
- Periodic trends are the patterns in the properties of elements that can be observed when moving across or down the periodic table.
- Some periodic trends include the trend in atomic radius, the trend in ionization energy, and the trend in electronegativity.
- Periodic trends can be used to predict the properties of an element based on its position in the periodic table.
General Inquiries
What are the key concepts covered in Chemistry: The Molecular Nature of Matter and Change, 9th Edition?
This comprehensive text covers fundamental principles, matter and energy, chemical bonding, the periodic table, chemical reactions, stoichiometry, states of matter, intermolecular forces, solutions, thermodynamics, kinetics, equilibrium, acids, bases, salts, and nuclear chemistry.
How is the periodic table organized, and what insights does it provide?
The periodic table organizes elements based on atomic number, revealing patterns in their properties and reactivities. It enables predictions about element behavior and facilitates the understanding of chemical reactions.
What are the different types of chemical reactions, and how are they classified?
Chemical reactions are classified into various types, including combination, decomposition, single displacement, double displacement, and combustion reactions. Each type involves specific changes in the arrangement of atoms and molecules.
How do intermolecular forces influence the properties of matter?
Intermolecular forces, such as van der Waals forces, hydrogen bonding, and dipole-dipole interactions, determine the physical properties of matter, including melting point, boiling point, and solubility.
What is the role of thermodynamics in predicting the spontaneity of chemical reactions?
Thermodynamics provides criteria to predict whether a chemical reaction will occur spontaneously. Entropy, enthalpy, and free energy are key concepts in determining reaction spontaneity.