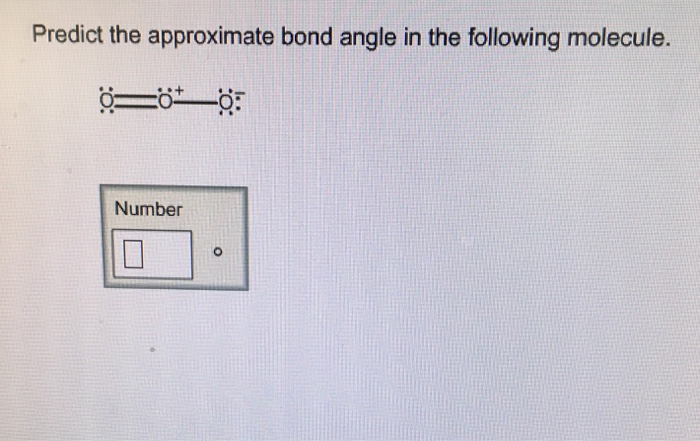
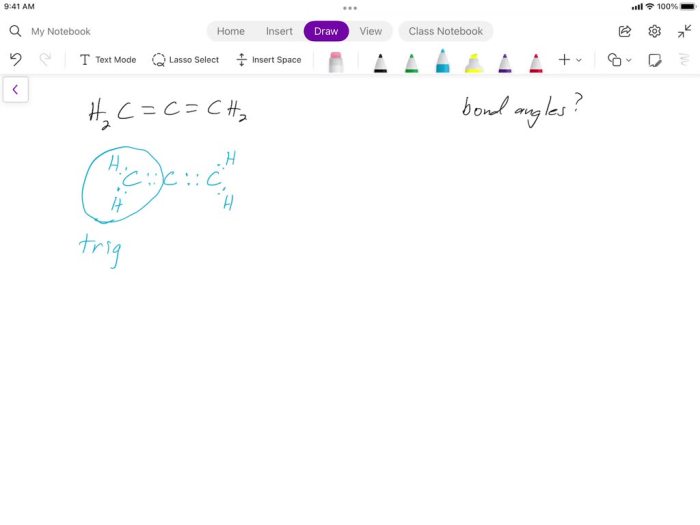
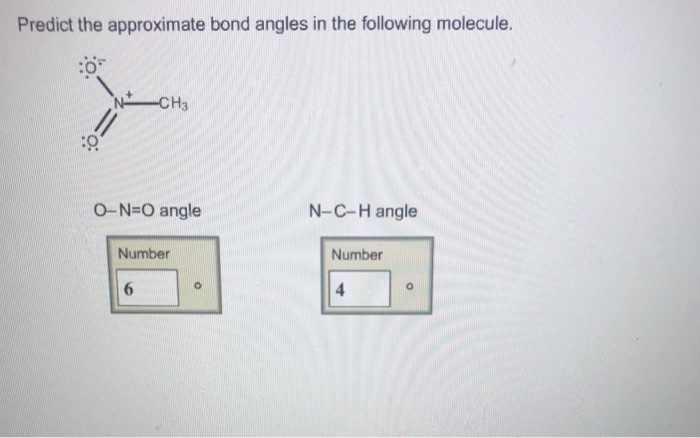
Predict the approximate bond angle in the molecule – Predicting bond angles is a fundamental aspect of understanding molecular structure. It unveils the spatial arrangement of atoms within a molecule, providing insights into its properties and behavior.
This comprehensive guide delves into the methods, factors, and applications of bond angle prediction, empowering readers to decipher the intricacies of molecular geometry.
Methods for Predicting Bond Angles
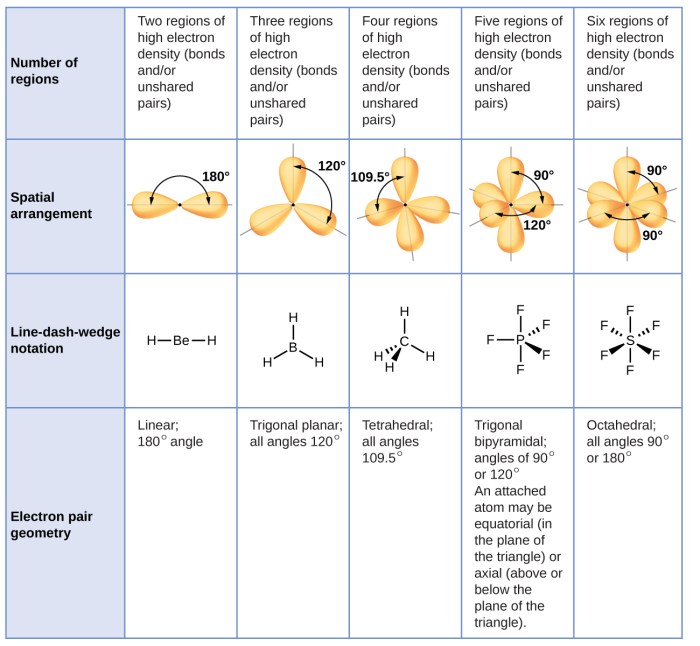
Valence Shell Electron Pair Repulsion (VSEPR) Theory, Predict the approximate bond angle in the molecule
The VSEPR theory is a simple and widely used method for predicting bond angles based on the number of valence electron pairs around the central atom. The theory states that electron pairs repel each other, and the molecular geometry will adopt the arrangement that minimizes this repulsion.
The VSEPR theory can be used to predict the bond angles of molecules with a variety of shapes, including linear, trigonal planar, tetrahedral, and octahedral.
Hybridization
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals that have different shapes and energies. The type of hybridization that occurs depends on the number and type of valence electrons on the central atom. Hybridization can affect bond angles by changing the shape of the electron orbitals involved in bonding.
For example, sp 3hybridization results in tetrahedral bond angles, while sp 2hybridization results in trigonal planar bond angles.
Other Methods
In addition to VSEPR theory and hybridization, there are other methods for predicting bond angles, such as:
- Molecular orbital theory
- Density functional theory
- Hartree-Fock theory
FAQ Section: Predict The Approximate Bond Angle In The Molecule
What factors influence bond angles?
Factors influencing bond angles include electronegativity, lone pairs, and steric hindrance.
How is VSEPR theory used to predict bond angles?
VSEPR theory predicts bond angles based on the repulsion between electron pairs, resulting in specific molecular shapes.
What are the limitations of bond angle prediction methods?
Limitations include the accuracy of experimental data, the complexity of molecules, and the approximations used in theoretical models.