Embark on a captivating journey into the realm of properties of covalent bonds pogil, where we unravel the intricate dance of shared electrons and delve into the fundamental characteristics that govern the molecular world.
Covalent bonds, the cornerstone of countless molecules, exhibit a fascinating array of properties that dictate their behavior and influence the very nature of matter. Join us as we explore the strength, polarity, and energetics of these chemical bonds, unraveling the secrets that underpin their significance in chemistry, biology, and beyond.
Introduction to Covalent Bonds
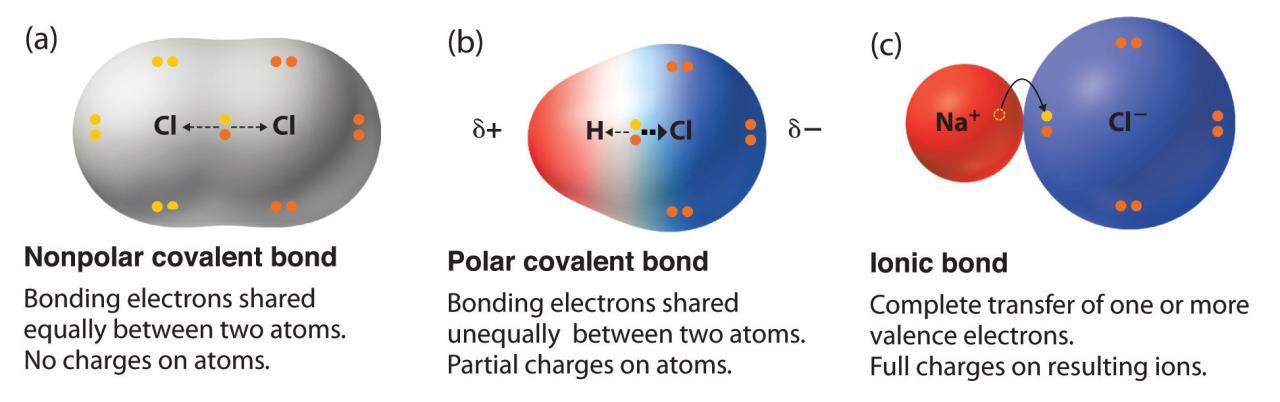
Covalent bonds are chemical bonds that involve the sharing of electron pairs between atoms. These bonds are formed when atoms come close enough to each other that their atomic orbitals overlap. The overlapping orbitals contain unpaired electrons, which are attracted to each other.
This attraction creates a covalent bond between the atoms.
Covalent bonds are the strongest type of chemical bond. They are found in many different types of molecules, including water, methane, and carbon dioxide. Covalent bonds can also be found in solids, such as diamond and graphite.
Types of Atoms that Can Form Covalent Bonds, Properties of covalent bonds pogil
Not all atoms can form covalent bonds. In general, only atoms of nonmetals can form covalent bonds. Nonmetals are elements that are located on the right side of the periodic table. They have high electronegativity, which means that they have a strong attraction for electrons.
The most common type of covalent bond is the single bond. A single bond is formed when two atoms share one pair of electrons. Double bonds and triple bonds are also possible. A double bond is formed when two atoms share two pairs of electrons, and a triple bond is formed when two atoms share three pairs of electrons.
Properties of Covalent Bonds
Covalent bonds form when atoms share one or more pairs of electrons. The strength and polarity of covalent bonds depend on the electronegativity of the atoms involved, the number of shared electrons, and the bond length.
Strength of Covalent Bonds
The strength of a covalent bond is determined by the bond energy, which is the amount of energy required to break the bond. Bond energy increases with increasing electronegativity difference between the atoms involved. This is because the more electronegative an atom, the more strongly it attracts electrons, which leads to a stronger bond.
Bond energy also increases with increasing bond order. Bond order is the number of shared electron pairs between two atoms. The higher the bond order, the stronger the bond.
Polarity of Covalent Bonds
A covalent bond is polar if the electrons are not shared equally between the atoms. This occurs when the atoms involved have different electronegativities. The more electronegative atom will have a greater share of the electrons, resulting in a partial negative charge.
The less electronegative atom will have a partial positive charge.
The polarity of a covalent bond can be measured by the dipole moment, which is a vector that points from the positive end of the bond to the negative end.
Bond Length and Bond Energy
Bond length is the distance between the nuclei of the two atoms involved in a covalent bond. Bond length decreases with increasing bond energy. This is because the stronger the bond, the closer the atoms are held together.
Number of Shared Electrons and Bond Strength
The number of shared electrons between two atoms affects the strength of the covalent bond. The more shared electrons, the stronger the bond. This is because the more shared electrons there are, the more electron density there is between the atoms, which leads to a stronger electrostatic attraction.
Examples of Covalent Bonds
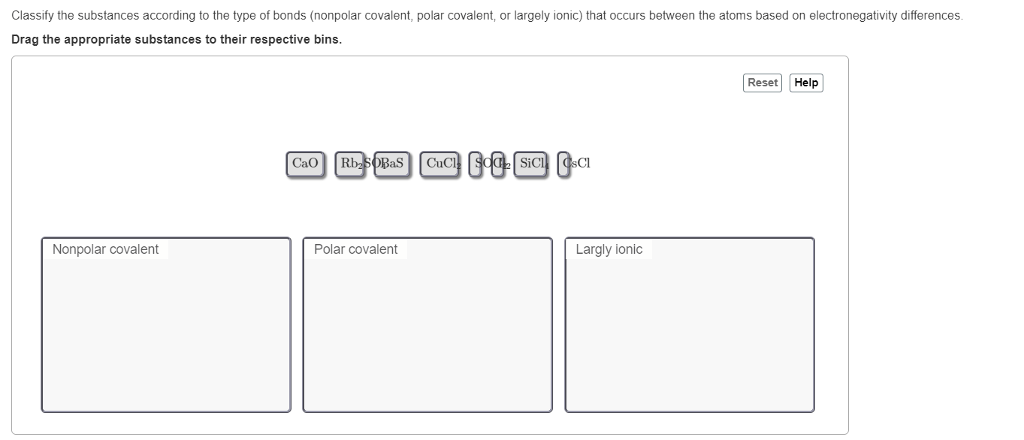
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. This sharing results in the formation of molecules with unique properties that depend on the nature of the covalent bonds.
Water
Water (H 2O) is a molecule composed of two hydrogen atoms covalently bonded to an oxygen atom. The covalent bonds in water are polar, meaning that the electrons are not shared equally between the atoms. This polarity gives water its unique properties, such as its high surface tension and ability to dissolve many substances.
Methane
Methane (CH 4) is a molecule composed of a carbon atom covalently bonded to four hydrogen atoms. The covalent bonds in methane are nonpolar, meaning that the electrons are shared equally between the atoms. This nonpolarity gives methane its low solubility in water and its ability to act as a fuel.
Carbon Dioxide
Carbon dioxide (CO 2) is a molecule composed of a carbon atom covalently bonded to two oxygen atoms. The covalent bonds in carbon dioxide are polar, but less polar than the bonds in water. This polarity gives carbon dioxide its ability to dissolve in water and its role as a greenhouse gas.
Macromolecules
Covalent bonds also play a crucial role in the formation of macromolecules, such as proteins and DNA. In proteins, amino acids are linked together by covalent bonds to form long chains. These chains fold into specific shapes, which determine the protein’s function.
In DNA, nucleotides are linked together by covalent bonds to form a double helix. This double helix contains the genetic information necessary for life.
Applications of Covalent Bonds
Covalent bonds are the foundation of modern chemistry and play a crucial role in various scientific fields. Their unique properties make them essential for understanding and manipulating the behavior of atoms and molecules.
In chemistry, covalent bonds are responsible for the formation of molecules and compounds. They determine the structure, reactivity, and properties of chemical substances. By understanding the principles of covalent bonding, chemists can design and synthesize new materials with tailored properties.
Biology
In biology, covalent bonds are the building blocks of life. They hold together the complex molecules that make up cells, tissues, and organs. The specific arrangement of atoms and the strength of covalent bonds determine the structure and function of biological molecules, such as proteins, carbohydrates, and nucleic acids.
Materials Science
In materials science, covalent bonds are responsible for the properties of solids, liquids, and gases. The strength and arrangement of covalent bonds determine the hardness, elasticity, and electrical conductivity of materials. By controlling the covalent bonding in materials, scientists can design and develop new materials with improved properties for various applications.
Nanotechnology
In nanotechnology, covalent bonds are used to create and manipulate materials at the nanoscale. By precisely controlling the formation of covalent bonds, scientists can create nanostructures with unique properties and applications. These nanostructures have the potential to revolutionize fields such as electronics, medicine, and energy.
Biotechnology
In biotechnology, covalent bonds are used to modify and engineer biological systems. By manipulating the covalent bonds in DNA, scientists can create genetically modified organisms with specific traits. This technology has applications in agriculture, medicine, and environmental science.
Popular Questions: Properties Of Covalent Bonds Pogil
What are the key factors that determine the strength of a covalent bond?
The strength of a covalent bond is primarily influenced by the number of shared electrons, the electronegativity of the bonded atoms, and the bond length.
How do covalent bonds contribute to the formation of macromolecules?
Covalent bonds are the primary force responsible for linking atoms together to form larger molecules, including complex biomolecules such as proteins and DNA.
What are some practical applications of covalent bonds in everyday life?
Covalent bonds play a crucial role in various technologies and products, including plastics, pharmaceuticals, and semiconductors, shaping our modern world.